Secondary batteries that are capable of repeated charge and discharge, high efficiency, and strong environmental adaptability are important research directions for energy storage technologies. Lithium-ion batteries generally use organic electrolytes to achieve a wide electrochemical window of more than 3 V, and therefore have a higher energy density than water-based ion batteries. However, organic electrolytes are not only toxic but also flammable. If they are used improperly, they can cause serious safety and environmental problems, which restricts the application of lithium-ion batteries in large-scale energy storage. Researchers have been trying to replace organic electrolytes with aqueous electrolytes. Relatively speaking, aqueous electrolytes are environmentally friendly and safe, and their ionic conductivity is two orders of magnitude higher than that of organic electrolytes, which is expected to achieve high power of the battery, avoiding the strict manufacturing conditions required for organic electrolytes, and greatly reducing production costs. Therefore, water-based ion batteries have important application prospects in the large-scale energy storage field at the grid level.
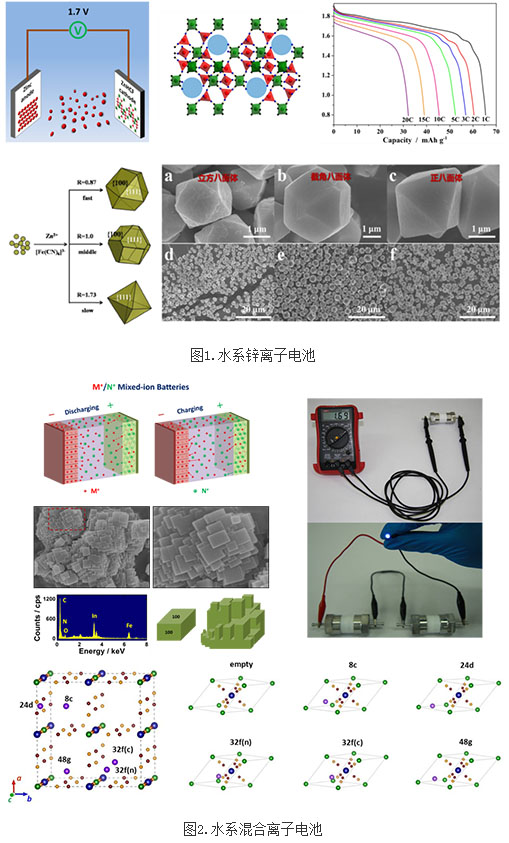
The working voltage of water-based ion batteries is relatively low (usually less than 1.5V), so the energy density is difficult to increase, which restricts the development of water-based ion batteries. Focusing on this issue, the research team of Liu Zhaoping, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, has carried out exploration and research on new high-voltage water-based ion batteries in recent years, and has made a series of progress. In 2013, the research team proposed a new concept of "M+/N+ mixed ions" to construct two aqueous ion cells, Na0.44MnO2/TiP2O7 and LiMn2O4/Na0.22MnO2 (Scientific Reports 2013, 3, 1946). The operation of this type of battery relies on the migration of bimetallic ions, unlike the traditional rocking chair type metal ion battery that only operates on a metal ion. This concept has enriched the theory of secondary batteries and opened up new directions for the development of secondary batteries. Subsequently, the research team used this new concept, using three-dimensional frame structure materials for the electrode positive and negative electrode materials, and developed a new system of aqueous secondary battery Ni1Zn1HCF/TiP2O7 and Ni1Zn1HCF/NaTi2(PO4)3 with an operating voltage of >1.2 V, and displayed Good application prospects (ChemSusChem 2014, 7, 2295). In addition, the research team found that rhombohedral zinc ferrocyanide complex ZnHCF can be used as a cathode material for zinc ion batteries, and construct a water-based zinc-ion battery with an operating voltage of 1.7V and an energy density of 100 Wh/kg with a metal zinc anode. Energy Materials 2015, 5, 1400930). Furthermore, the ZnHCF microparticles with cubic, truncated and regular octahedron morphology were prepared using the "crystal-regulating-growth" strategy, revealing that the electrochemical properties of the ZnHCF microparticles have a significant relationship with the particle morphology (Scientific Reports 2015, 5, 18263 ). The results of this series of studies provide new ideas for the development of high voltage aqueous ion batteries.
Recently, the research team used a novel Prussian blue compound InHCF as the positive electrode, sodium titanium phosphate NaTi2 (PO4) 3 and titanium pyrophosphate TiP2O7 as the negative electrode, and mixed aqueous alkali metal ions as electrolytes to construct a series of high-voltage water-based batteries. Among them, the InHCF/NaTi2(PO4)3 battery has an operating voltage of 1.6 V, an energy density of 56 Wh/kg, and a power density of over 2700 W/kg. In order to reveal the mechanism of water regulation of alkali metal ion insertion in InHCF, Chen Liang and Shao Hezhuo conducted in-depth theoretical calculations and simulations. It was found that for lithium/sodium ions, water molecules can increase their insertion/demonstation potential and reduce their embedding reaction. Kinetics, for potassium ions, water molecules do not participate in the embedding reaction. The research work was recently published in Nature Communications 2016, 7, 11982, and highly reviewed by reviewers. This research work not only has important theoretical significance for designing high specific energy electrode materials and understanding the energy storage mechanism of ion-embedded electrode materials, but also lays a scientific foundation for the establishment of practical water-based ion batteries.
The above research work was supported by the National Natural Science Foundation of China Youth Program (51404233), the Key Deployment Project of the Chinese Academy of Sciences (KGZD-EW-T08-2), the Natural Science Foundation of Zhejiang Province (LY15B030004) and the Ningbo Natural Science Foundation (2014A610044).
Galvanized Mild Steel Link Chain
Mild Steel Welded Straight Link Chain,Galvanised Steel Long Link Chain ,Mild Steel Galvanized Link Chain,Mild Steel Link Chain
Guangdong Gongyou Lift Slings Machinery CO.,LTD , https://www.gongyouslings.com