1. Ideal Weyl points and helicoid surface states in artificial photonic crystal structures. Material Name: Artificial Photonic Crystal Research Team: University of Birmingham, Shenzhen University and Institute of Physics, Chinese Academy of Sciences Weyl The point is the intersection of the linearly dispersed energy band of the three-dimensional crystal, which provides an opportunity to explore various wonderful phenomena, such as topologically protected surface states and chiral anomalies. However, the Weyl points are all present at the same energy and are still lacking in any other Weyl system with separate energy bands, which poses a serious limitation to the further development of Weyl physics and potential applications. Through the experimental characterization of saddle-shaped metal spiral microwave photonic crystals, Yang et al. observed the ideal Weyl points related to each other through symmetry operation and exhibited the topological surface state of the spiral structure. This system provides a photonic platform for exploring ideal Weyl systems and developing possible topological equipment. (Science DOI: 10.1126/science.aaq1221) 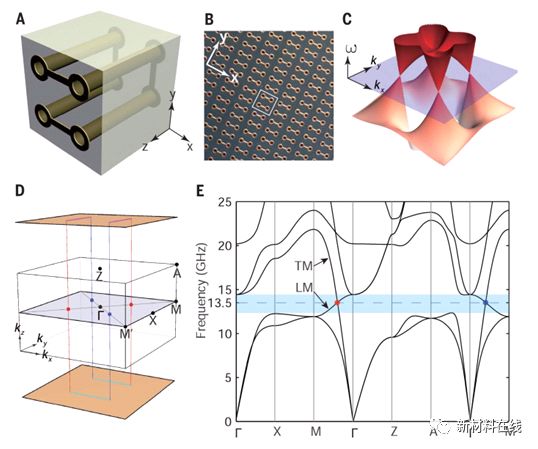 2. Acoustically modulated magnetic resonance imaging of gas-filled protein nanostructures. Material Name: Gas-filled Protein Nanostructures Research Team: Non-invasive Bioimaging Institute, Shapiro Research Group, California Institute of Technology The materials needed should be able to interact with energy in the form of deep penetration, such as magnetic fields and sound waves. Lu et al. demonstrated a balloon (GV), a unique gas-filled protein nanostructure that has a differential magnetic susceptibility relative to water, producing intense magnetic resonance imaging (MRI) contrast at subnanomolar concentrations, and Contrast can be eliminated using in situ ultrasound to achieve backgroundless imaging. The ability of these nanostructures to serve as genetically encoded reporters was demonstrated in vitro, in vivo, and in three in vivo scenarios. Different genetic variants of GV have different magnetic or mechanical phenotypes, enabling multiplexed imaging using parametric MRI and differential acoustic sensitivity. In addition, cluster-induced MRI contrast changes make the design of dynamic molecular sensors possible. By combining the complementary physics of MRI and ultrasound, this nanomaterial has a unique molecular imaging approach with special advantages and capabilities. (Nature Materials DOI: 10.1038/s41563-018-0023-7)
2. Acoustically modulated magnetic resonance imaging of gas-filled protein nanostructures. Material Name: Gas-filled Protein Nanostructures Research Team: Non-invasive Bioimaging Institute, Shapiro Research Group, California Institute of Technology The materials needed should be able to interact with energy in the form of deep penetration, such as magnetic fields and sound waves. Lu et al. demonstrated a balloon (GV), a unique gas-filled protein nanostructure that has a differential magnetic susceptibility relative to water, producing intense magnetic resonance imaging (MRI) contrast at subnanomolar concentrations, and Contrast can be eliminated using in situ ultrasound to achieve backgroundless imaging. The ability of these nanostructures to serve as genetically encoded reporters was demonstrated in vitro, in vivo, and in three in vivo scenarios. Different genetic variants of GV have different magnetic or mechanical phenotypes, enabling multiplexed imaging using parametric MRI and differential acoustic sensitivity. In addition, cluster-induced MRI contrast changes make the design of dynamic molecular sensors possible. By combining the complementary physics of MRI and ultrasound, this nanomaterial has a unique molecular imaging approach with special advantages and capabilities. (Nature Materials DOI: 10.1038/s41563-018-0023-7) 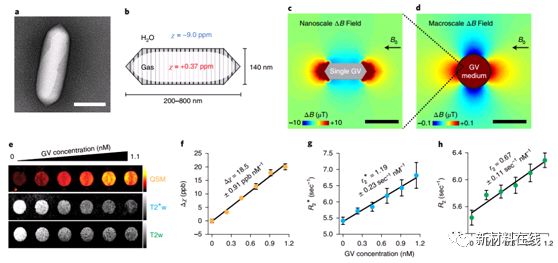 3. Insight into doping efficiency of organic semiconductors from the analysis of the density of states in n-doped C60 and ZnPc by analyzing the density of states in n-doped C60 and ZnPc. Organic semiconductor research team: The Dotmann research group of the Dresden University of Technology in Germany plays a crucial role in semiconductor physics, where n-doping is controlled by the ionization energy of the impurity relative to the edge of the conduction band. In organic semiconductors, high-efficiency doping is mainly affected by various effects that are not yet fully understood. Gaul et al. used the simulations and experiments of forward and reverse photoelectron spectroscopy to measure the state density and cost of the prototype material C60 and zinc phthalocyanine n-doped by high-efficiency benzimidazolyl (2-Cyc-DMBI). Meter level position. They also studied the effect of the gap state caused by doping, in particular the effect of the difference Δ1 between the electron affinity of the undoped material and the ionization potential of its doped counterpart. Studies have shown that this parameter is critical to the generation of free carriers and affects the conductivity of the doped film. Adjusting Δ1 may provide an alternative strategy for optimizing the electronic properties of organic semiconductors. (Nature Materials DOI: 10.1038/s41563-018-0030-8)
3. Insight into doping efficiency of organic semiconductors from the analysis of the density of states in n-doped C60 and ZnPc by analyzing the density of states in n-doped C60 and ZnPc. Organic semiconductor research team: The Dotmann research group of the Dresden University of Technology in Germany plays a crucial role in semiconductor physics, where n-doping is controlled by the ionization energy of the impurity relative to the edge of the conduction band. In organic semiconductors, high-efficiency doping is mainly affected by various effects that are not yet fully understood. Gaul et al. used the simulations and experiments of forward and reverse photoelectron spectroscopy to measure the state density and cost of the prototype material C60 and zinc phthalocyanine n-doped by high-efficiency benzimidazolyl (2-Cyc-DMBI). Meter level position. They also studied the effect of the gap state caused by doping, in particular the effect of the difference Δ1 between the electron affinity of the undoped material and the ionization potential of its doped counterpart. Studies have shown that this parameter is critical to the generation of free carriers and affects the conductivity of the doped film. Adjusting Δ1 may provide an alternative strategy for optimizing the electronic properties of organic semiconductors. (Nature Materials DOI: 10.1038/s41563-018-0030-8) 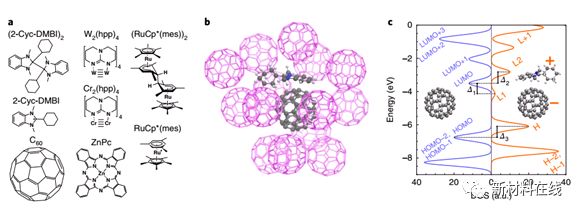 4. The vibration excited molecules adsorption and desorption on the metal surface observation (Observation of the adsorption and desorption of vibrationally excited molecules on a metal surface) Material Name: gold team: Surface Chemical Wodtke Study Group The most common catalytic University of Gottingen, Germany The mechanisms are Langmuir and Hinshelwood (LH). In the LH mechanism, the reactants are first adsorbed and then reacted with surface heating. The vibration (relaxation) lifetime measured for molecules adsorbed on the metal surface is in the range of a few picoseconds. Therefore, in addition to the LH reaction which occurs when the intermediate is adsorbed by molecular physics, vibrational acceleration of LH chemistry is rarely observed. Shirhatti et al. directly tested the adsorption and subsequent desorption of CO molecules excited by Au(111) surface vibration. The results show that CO(v = 1) exists on the Au(111) surface for about 1 x 10-10 s. The long life of the sorbent on the metal surface is surprisingly unexpected, presenting an interesting challenge to the current understanding of the vibrational energy dissipation on metal surfaces. This also suggests that the vibrational acceleration of surface chemistry may be more prevalent than is generally believed. (Nature Chemistry DOI: 10.1038/s41557-018-0003-1)
4. The vibration excited molecules adsorption and desorption on the metal surface observation (Observation of the adsorption and desorption of vibrationally excited molecules on a metal surface) Material Name: gold team: Surface Chemical Wodtke Study Group The most common catalytic University of Gottingen, Germany The mechanisms are Langmuir and Hinshelwood (LH). In the LH mechanism, the reactants are first adsorbed and then reacted with surface heating. The vibration (relaxation) lifetime measured for molecules adsorbed on the metal surface is in the range of a few picoseconds. Therefore, in addition to the LH reaction which occurs when the intermediate is adsorbed by molecular physics, vibrational acceleration of LH chemistry is rarely observed. Shirhatti et al. directly tested the adsorption and subsequent desorption of CO molecules excited by Au(111) surface vibration. The results show that CO(v = 1) exists on the Au(111) surface for about 1 x 10-10 s. The long life of the sorbent on the metal surface is surprisingly unexpected, presenting an interesting challenge to the current understanding of the vibrational energy dissipation on metal surfaces. This also suggests that the vibrational acceleration of surface chemistry may be more prevalent than is generally believed. (Nature Chemistry DOI: 10.1038/s41557-018-0003-1) 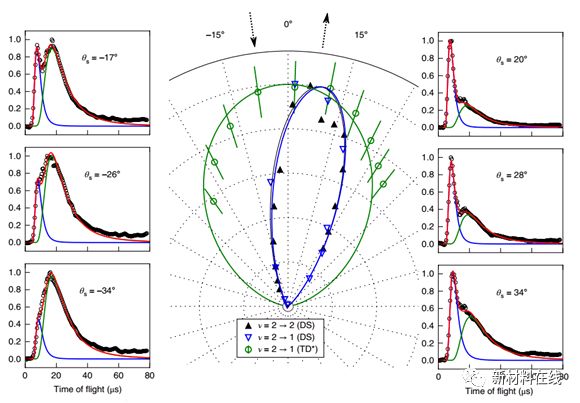 5. Solvent-enabled control of reactivity for liquid-phase reactions of biomass-derived compounds. Material Name: Biomass Derivatives Research Team: University of Wisconsin Madison The campus's Dumesic research group can achieve high rates and high selectivity by using organic solvents in biomass conversion reactions. Mellmer et al. elucidate the effect of organic solvent mixtures and water on the kinetics of acid-catalyzed dehydration reactions associated with biomass conversion. Based on the results of reaction kinetic studies, combined with classical and ab initio molecular dynamics simulations, it was shown that by varying the degree of solvation of the initial and transition states of these catalytic processes, the rate of acid-catalyzed reactions in the liquid phase can be increased. As the number of ortho-hydroxy groups or oxygen-containing groups in the reactants increases, the extent of these effects increases, from alcohol (butanol) to diol (1,2-propanediol), to carbohydrates (fructose). ). An understanding of these solvent effects can be used to optimize the rate and selectivity of the molecular hydroxymethylfurfural from the fructose-producing material platform. (Nature Catalysis DOI: 10.1038/s41929-018-0027-3)
5. Solvent-enabled control of reactivity for liquid-phase reactions of biomass-derived compounds. Material Name: Biomass Derivatives Research Team: University of Wisconsin Madison The campus's Dumesic research group can achieve high rates and high selectivity by using organic solvents in biomass conversion reactions. Mellmer et al. elucidate the effect of organic solvent mixtures and water on the kinetics of acid-catalyzed dehydration reactions associated with biomass conversion. Based on the results of reaction kinetic studies, combined with classical and ab initio molecular dynamics simulations, it was shown that by varying the degree of solvation of the initial and transition states of these catalytic processes, the rate of acid-catalyzed reactions in the liquid phase can be increased. As the number of ortho-hydroxy groups or oxygen-containing groups in the reactants increases, the extent of these effects increases, from alcohol (butanol) to diol (1,2-propanediol), to carbohydrates (fructose). ). An understanding of these solvent effects can be used to optimize the rate and selectivity of the molecular hydroxymethylfurfural from the fructose-producing material platform. (Nature Catalysis DOI: 10.1038/s41929-018-0027-3) 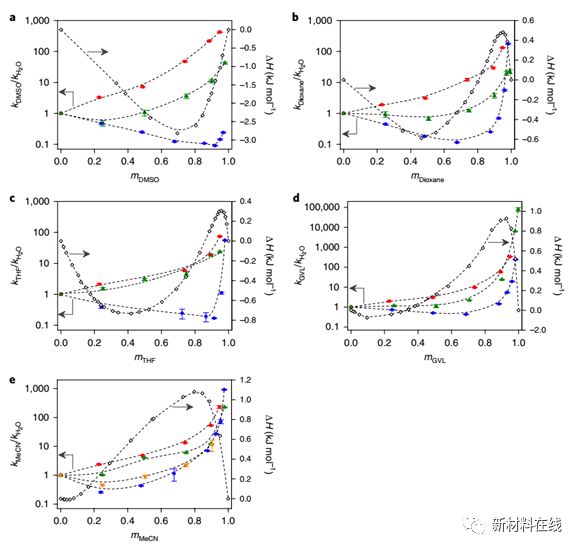 6. Spatially controlled doping of two-dimensional SnS2 through intercalation for electronics. Material Name: Two-Dimensional SnS2 Research Team: Stanford University Cui Wei Research Group Doped Semiconductor is Modern The most important building element of an electronic device. In silicon-based integrated circuits, simple, controlled manufacturing and integration of these materials can be achieved without introducing high-resistance interfaces. In addition, the advent of two-dimensional (2D) materials has enabled atomic-scale thin integrated circuits to be realized. However, the 2D nature of these materials hinders carrier doping using conventional ion implantation techniques and further hinders device development. Gong et al. demonstrated a solvent-based intercalation method for p-type doping, n-type doping, and degenerate doping of semiconductors in the same parent metal at atomic limits. In contrast to the naturally grown n-type S-vacancy SnS2, the Cu intercalation double-layer SnS2 obtained by this technique exhibits a hole field effect mobility of about 40 cm2V-1s-1, and the obtained Co-SnS2 exhibits a metal. Chemical properties, surface resistance is comparable to several layers of graphene. Combining this intercalation technique with lithography further enables atomic-scale, seamless pn metal junctions with precise spatial and dimensional control, which enables in-plane heterojunctions for practical availability for integrated devices and other 2D materials. . Thus, this insertion method provides a new way to connect two different worlds of integrated circuits and atomic thin materials. (Nature Nanotechnology DOI: 10.1038/s41565-018-0069-3)
6. Spatially controlled doping of two-dimensional SnS2 through intercalation for electronics. Material Name: Two-Dimensional SnS2 Research Team: Stanford University Cui Wei Research Group Doped Semiconductor is Modern The most important building element of an electronic device. In silicon-based integrated circuits, simple, controlled manufacturing and integration of these materials can be achieved without introducing high-resistance interfaces. In addition, the advent of two-dimensional (2D) materials has enabled atomic-scale thin integrated circuits to be realized. However, the 2D nature of these materials hinders carrier doping using conventional ion implantation techniques and further hinders device development. Gong et al. demonstrated a solvent-based intercalation method for p-type doping, n-type doping, and degenerate doping of semiconductors in the same parent metal at atomic limits. In contrast to the naturally grown n-type S-vacancy SnS2, the Cu intercalation double-layer SnS2 obtained by this technique exhibits a hole field effect mobility of about 40 cm2V-1s-1, and the obtained Co-SnS2 exhibits a metal. Chemical properties, surface resistance is comparable to several layers of graphene. Combining this intercalation technique with lithography further enables atomic-scale, seamless pn metal junctions with precise spatial and dimensional control, which enables in-plane heterojunctions for practical availability for integrated devices and other 2D materials. . Thus, this insertion method provides a new way to connect two different worlds of integrated circuits and atomic thin materials. (Nature Nanotechnology DOI: 10.1038/s41565-018-0069-3) 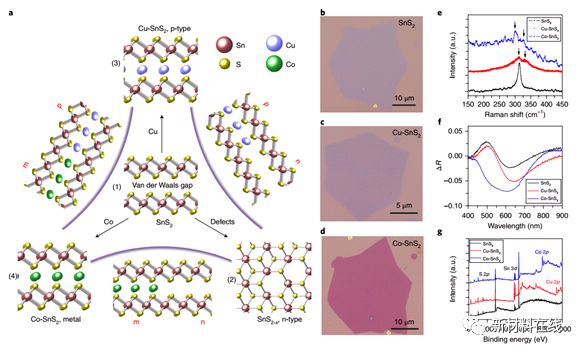 7. The role of reticular chemistry in the design of CO2 reduction catalysts. Material Name: Framework Compound Research Team: Yaghi Research Group, University of California, Berkeley, currently the most advanced for carbon dioxide photoreduction A problem with electroreduced catalysts remains that no single system can be independently controlled and thereby optimize the interaction between activity, selectivity and efficiency. Frame chemistry is believed to control the chemical and structural characteristics (activity and selectivity) of porous crystalline materials and the output photoelectron properties (efficiency) with atomic precision. The molecular building blocks in the selection framework chemist's toolbox enable rational design of the structure, the framework chemistry to integrate the catalytically active components, and the manner in which these building blocks are connected imparts the optoelectronic properties of the desired material. The fact that these aspects can be fine-tuned independently demonstrates the prospects for framework chemistry and contributes to the design of the next generation of carbon dioxide reduction catalysts. (Nature Materials DOI: 10.1038/s41563-018-0033-5)
7. The role of reticular chemistry in the design of CO2 reduction catalysts. Material Name: Framework Compound Research Team: Yaghi Research Group, University of California, Berkeley, currently the most advanced for carbon dioxide photoreduction A problem with electroreduced catalysts remains that no single system can be independently controlled and thereby optimize the interaction between activity, selectivity and efficiency. Frame chemistry is believed to control the chemical and structural characteristics (activity and selectivity) of porous crystalline materials and the output photoelectron properties (efficiency) with atomic precision. The molecular building blocks in the selection framework chemist's toolbox enable rational design of the structure, the framework chemistry to integrate the catalytically active components, and the manner in which these building blocks are connected imparts the optoelectronic properties of the desired material. The fact that these aspects can be fine-tuned independently demonstrates the prospects for framework chemistry and contributes to the design of the next generation of carbon dioxide reduction catalysts. (Nature Materials DOI: 10.1038/s41563-018-0033-5) 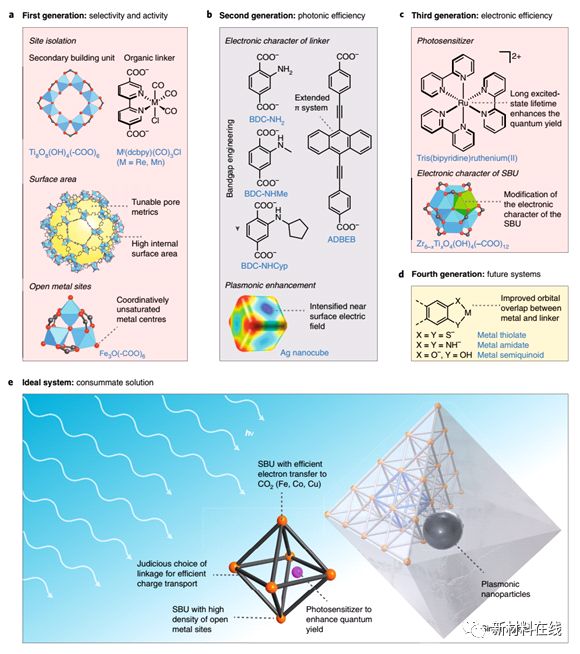
 2. Acoustically modulated magnetic resonance imaging of gas-filled protein nanostructures. Material Name: Gas-filled Protein Nanostructures Research Team: Non-invasive Bioimaging Institute, Shapiro Research Group, California Institute of Technology The materials needed should be able to interact with energy in the form of deep penetration, such as magnetic fields and sound waves. Lu et al. demonstrated a balloon (GV), a unique gas-filled protein nanostructure that has a differential magnetic susceptibility relative to water, producing intense magnetic resonance imaging (MRI) contrast at subnanomolar concentrations, and Contrast can be eliminated using in situ ultrasound to achieve backgroundless imaging. The ability of these nanostructures to serve as genetically encoded reporters was demonstrated in vitro, in vivo, and in three in vivo scenarios. Different genetic variants of GV have different magnetic or mechanical phenotypes, enabling multiplexed imaging using parametric MRI and differential acoustic sensitivity. In addition, cluster-induced MRI contrast changes make the design of dynamic molecular sensors possible. By combining the complementary physics of MRI and ultrasound, this nanomaterial has a unique molecular imaging approach with special advantages and capabilities. (Nature Materials DOI: 10.1038/s41563-018-0023-7)
2. Acoustically modulated magnetic resonance imaging of gas-filled protein nanostructures. Material Name: Gas-filled Protein Nanostructures Research Team: Non-invasive Bioimaging Institute, Shapiro Research Group, California Institute of Technology The materials needed should be able to interact with energy in the form of deep penetration, such as magnetic fields and sound waves. Lu et al. demonstrated a balloon (GV), a unique gas-filled protein nanostructure that has a differential magnetic susceptibility relative to water, producing intense magnetic resonance imaging (MRI) contrast at subnanomolar concentrations, and Contrast can be eliminated using in situ ultrasound to achieve backgroundless imaging. The ability of these nanostructures to serve as genetically encoded reporters was demonstrated in vitro, in vivo, and in three in vivo scenarios. Different genetic variants of GV have different magnetic or mechanical phenotypes, enabling multiplexed imaging using parametric MRI and differential acoustic sensitivity. In addition, cluster-induced MRI contrast changes make the design of dynamic molecular sensors possible. By combining the complementary physics of MRI and ultrasound, this nanomaterial has a unique molecular imaging approach with special advantages and capabilities. (Nature Materials DOI: 10.1038/s41563-018-0023-7)  3. Insight into doping efficiency of organic semiconductors from the analysis of the density of states in n-doped C60 and ZnPc by analyzing the density of states in n-doped C60 and ZnPc. Organic semiconductor research team: The Dotmann research group of the Dresden University of Technology in Germany plays a crucial role in semiconductor physics, where n-doping is controlled by the ionization energy of the impurity relative to the edge of the conduction band. In organic semiconductors, high-efficiency doping is mainly affected by various effects that are not yet fully understood. Gaul et al. used the simulations and experiments of forward and reverse photoelectron spectroscopy to measure the state density and cost of the prototype material C60 and zinc phthalocyanine n-doped by high-efficiency benzimidazolyl (2-Cyc-DMBI). Meter level position. They also studied the effect of the gap state caused by doping, in particular the effect of the difference Δ1 between the electron affinity of the undoped material and the ionization potential of its doped counterpart. Studies have shown that this parameter is critical to the generation of free carriers and affects the conductivity of the doped film. Adjusting Δ1 may provide an alternative strategy for optimizing the electronic properties of organic semiconductors. (Nature Materials DOI: 10.1038/s41563-018-0030-8)
3. Insight into doping efficiency of organic semiconductors from the analysis of the density of states in n-doped C60 and ZnPc by analyzing the density of states in n-doped C60 and ZnPc. Organic semiconductor research team: The Dotmann research group of the Dresden University of Technology in Germany plays a crucial role in semiconductor physics, where n-doping is controlled by the ionization energy of the impurity relative to the edge of the conduction band. In organic semiconductors, high-efficiency doping is mainly affected by various effects that are not yet fully understood. Gaul et al. used the simulations and experiments of forward and reverse photoelectron spectroscopy to measure the state density and cost of the prototype material C60 and zinc phthalocyanine n-doped by high-efficiency benzimidazolyl (2-Cyc-DMBI). Meter level position. They also studied the effect of the gap state caused by doping, in particular the effect of the difference Δ1 between the electron affinity of the undoped material and the ionization potential of its doped counterpart. Studies have shown that this parameter is critical to the generation of free carriers and affects the conductivity of the doped film. Adjusting Δ1 may provide an alternative strategy for optimizing the electronic properties of organic semiconductors. (Nature Materials DOI: 10.1038/s41563-018-0030-8)  4. The vibration excited molecules adsorption and desorption on the metal surface observation (Observation of the adsorption and desorption of vibrationally excited molecules on a metal surface) Material Name: gold team: Surface Chemical Wodtke Study Group The most common catalytic University of Gottingen, Germany The mechanisms are Langmuir and Hinshelwood (LH). In the LH mechanism, the reactants are first adsorbed and then reacted with surface heating. The vibration (relaxation) lifetime measured for molecules adsorbed on the metal surface is in the range of a few picoseconds. Therefore, in addition to the LH reaction which occurs when the intermediate is adsorbed by molecular physics, vibrational acceleration of LH chemistry is rarely observed. Shirhatti et al. directly tested the adsorption and subsequent desorption of CO molecules excited by Au(111) surface vibration. The results show that CO(v = 1) exists on the Au(111) surface for about 1 x 10-10 s. The long life of the sorbent on the metal surface is surprisingly unexpected, presenting an interesting challenge to the current understanding of the vibrational energy dissipation on metal surfaces. This also suggests that the vibrational acceleration of surface chemistry may be more prevalent than is generally believed. (Nature Chemistry DOI: 10.1038/s41557-018-0003-1)
4. The vibration excited molecules adsorption and desorption on the metal surface observation (Observation of the adsorption and desorption of vibrationally excited molecules on a metal surface) Material Name: gold team: Surface Chemical Wodtke Study Group The most common catalytic University of Gottingen, Germany The mechanisms are Langmuir and Hinshelwood (LH). In the LH mechanism, the reactants are first adsorbed and then reacted with surface heating. The vibration (relaxation) lifetime measured for molecules adsorbed on the metal surface is in the range of a few picoseconds. Therefore, in addition to the LH reaction which occurs when the intermediate is adsorbed by molecular physics, vibrational acceleration of LH chemistry is rarely observed. Shirhatti et al. directly tested the adsorption and subsequent desorption of CO molecules excited by Au(111) surface vibration. The results show that CO(v = 1) exists on the Au(111) surface for about 1 x 10-10 s. The long life of the sorbent on the metal surface is surprisingly unexpected, presenting an interesting challenge to the current understanding of the vibrational energy dissipation on metal surfaces. This also suggests that the vibrational acceleration of surface chemistry may be more prevalent than is generally believed. (Nature Chemistry DOI: 10.1038/s41557-018-0003-1)  5. Solvent-enabled control of reactivity for liquid-phase reactions of biomass-derived compounds. Material Name: Biomass Derivatives Research Team: University of Wisconsin Madison The campus's Dumesic research group can achieve high rates and high selectivity by using organic solvents in biomass conversion reactions. Mellmer et al. elucidate the effect of organic solvent mixtures and water on the kinetics of acid-catalyzed dehydration reactions associated with biomass conversion. Based on the results of reaction kinetic studies, combined with classical and ab initio molecular dynamics simulations, it was shown that by varying the degree of solvation of the initial and transition states of these catalytic processes, the rate of acid-catalyzed reactions in the liquid phase can be increased. As the number of ortho-hydroxy groups or oxygen-containing groups in the reactants increases, the extent of these effects increases, from alcohol (butanol) to diol (1,2-propanediol), to carbohydrates (fructose). ). An understanding of these solvent effects can be used to optimize the rate and selectivity of the molecular hydroxymethylfurfural from the fructose-producing material platform. (Nature Catalysis DOI: 10.1038/s41929-018-0027-3)
5. Solvent-enabled control of reactivity for liquid-phase reactions of biomass-derived compounds. Material Name: Biomass Derivatives Research Team: University of Wisconsin Madison The campus's Dumesic research group can achieve high rates and high selectivity by using organic solvents in biomass conversion reactions. Mellmer et al. elucidate the effect of organic solvent mixtures and water on the kinetics of acid-catalyzed dehydration reactions associated with biomass conversion. Based on the results of reaction kinetic studies, combined with classical and ab initio molecular dynamics simulations, it was shown that by varying the degree of solvation of the initial and transition states of these catalytic processes, the rate of acid-catalyzed reactions in the liquid phase can be increased. As the number of ortho-hydroxy groups or oxygen-containing groups in the reactants increases, the extent of these effects increases, from alcohol (butanol) to diol (1,2-propanediol), to carbohydrates (fructose). ). An understanding of these solvent effects can be used to optimize the rate and selectivity of the molecular hydroxymethylfurfural from the fructose-producing material platform. (Nature Catalysis DOI: 10.1038/s41929-018-0027-3)  6. Spatially controlled doping of two-dimensional SnS2 through intercalation for electronics. Material Name: Two-Dimensional SnS2 Research Team: Stanford University Cui Wei Research Group Doped Semiconductor is Modern The most important building element of an electronic device. In silicon-based integrated circuits, simple, controlled manufacturing and integration of these materials can be achieved without introducing high-resistance interfaces. In addition, the advent of two-dimensional (2D) materials has enabled atomic-scale thin integrated circuits to be realized. However, the 2D nature of these materials hinders carrier doping using conventional ion implantation techniques and further hinders device development. Gong et al. demonstrated a solvent-based intercalation method for p-type doping, n-type doping, and degenerate doping of semiconductors in the same parent metal at atomic limits. In contrast to the naturally grown n-type S-vacancy SnS2, the Cu intercalation double-layer SnS2 obtained by this technique exhibits a hole field effect mobility of about 40 cm2V-1s-1, and the obtained Co-SnS2 exhibits a metal. Chemical properties, surface resistance is comparable to several layers of graphene. Combining this intercalation technique with lithography further enables atomic-scale, seamless pn metal junctions with precise spatial and dimensional control, which enables in-plane heterojunctions for practical availability for integrated devices and other 2D materials. . Thus, this insertion method provides a new way to connect two different worlds of integrated circuits and atomic thin materials. (Nature Nanotechnology DOI: 10.1038/s41565-018-0069-3)
6. Spatially controlled doping of two-dimensional SnS2 through intercalation for electronics. Material Name: Two-Dimensional SnS2 Research Team: Stanford University Cui Wei Research Group Doped Semiconductor is Modern The most important building element of an electronic device. In silicon-based integrated circuits, simple, controlled manufacturing and integration of these materials can be achieved without introducing high-resistance interfaces. In addition, the advent of two-dimensional (2D) materials has enabled atomic-scale thin integrated circuits to be realized. However, the 2D nature of these materials hinders carrier doping using conventional ion implantation techniques and further hinders device development. Gong et al. demonstrated a solvent-based intercalation method for p-type doping, n-type doping, and degenerate doping of semiconductors in the same parent metal at atomic limits. In contrast to the naturally grown n-type S-vacancy SnS2, the Cu intercalation double-layer SnS2 obtained by this technique exhibits a hole field effect mobility of about 40 cm2V-1s-1, and the obtained Co-SnS2 exhibits a metal. Chemical properties, surface resistance is comparable to several layers of graphene. Combining this intercalation technique with lithography further enables atomic-scale, seamless pn metal junctions with precise spatial and dimensional control, which enables in-plane heterojunctions for practical availability for integrated devices and other 2D materials. . Thus, this insertion method provides a new way to connect two different worlds of integrated circuits and atomic thin materials. (Nature Nanotechnology DOI: 10.1038/s41565-018-0069-3)  7. The role of reticular chemistry in the design of CO2 reduction catalysts. Material Name: Framework Compound Research Team: Yaghi Research Group, University of California, Berkeley, currently the most advanced for carbon dioxide photoreduction A problem with electroreduced catalysts remains that no single system can be independently controlled and thereby optimize the interaction between activity, selectivity and efficiency. Frame chemistry is believed to control the chemical and structural characteristics (activity and selectivity) of porous crystalline materials and the output photoelectron properties (efficiency) with atomic precision. The molecular building blocks in the selection framework chemist's toolbox enable rational design of the structure, the framework chemistry to integrate the catalytically active components, and the manner in which these building blocks are connected imparts the optoelectronic properties of the desired material. The fact that these aspects can be fine-tuned independently demonstrates the prospects for framework chemistry and contributes to the design of the next generation of carbon dioxide reduction catalysts. (Nature Materials DOI: 10.1038/s41563-018-0033-5)
7. The role of reticular chemistry in the design of CO2 reduction catalysts. Material Name: Framework Compound Research Team: Yaghi Research Group, University of California, Berkeley, currently the most advanced for carbon dioxide photoreduction A problem with electroreduced catalysts remains that no single system can be independently controlled and thereby optimize the interaction between activity, selectivity and efficiency. Frame chemistry is believed to control the chemical and structural characteristics (activity and selectivity) of porous crystalline materials and the output photoelectron properties (efficiency) with atomic precision. The molecular building blocks in the selection framework chemist's toolbox enable rational design of the structure, the framework chemistry to integrate the catalytically active components, and the manner in which these building blocks are connected imparts the optoelectronic properties of the desired material. The fact that these aspects can be fine-tuned independently demonstrates the prospects for framework chemistry and contributes to the design of the next generation of carbon dioxide reduction catalysts. (Nature Materials DOI: 10.1038/s41563-018-0033-5) 
Laizhou Chenke trading Co., Ltd. , https://www.chenkegroup.com