The Ministry of Education, Culture, Sports, Science and Technology of Japan has formulated a research and development strategy for the reduction of greenhouse gases, and the Science and Technology Agency (JST) is advancing “Advanced Low Carbon Technology Development (ALCA)†under the guidance of the strategy. The project was held in February 2016. One of the development areas will be a "New Generation Battery" technical briefing. The Tohoku University and Kansai University of Japan introduced the development of a new basic technology for lithium-sulfur (LIS) batteries.
As a strong candidate for "post-lithium-ion batteries," LIS batteries are actively researched and developed. In this briefing session, a number of basic technologies being developed to realize LIS batteries were introduced. One of them is the solid electrolyte developed by a professor at the Advanced Research Institute of Atomic and Molecular Materials at Tohoku University in Japan and a R&D team under the guidance of lecturer Yu Genchi. Its electrolyte is complex hydride and is expected to be used in LIS batteries.
The LIS battery is a battery that uses sulfur as a positive electrode material and lithium metal as a negative electrode material. The theoretical capacity density of sulfur as a cathode material is about 1670 mAh/g, which is more than 6 times that of a commonly used ternary material for lithium ion battery cathode materials. In addition, the theoretical capacity density of metallic lithium as a negative electrode material is 3861 mAh/g, which is about 10 times that of a negative electrode material carbon (372 mAh/g) commonly used for lithium ion batteries. Energy density is expected to increase dramatically compared to current lithium-ion batteries.
However, the problem with the LIS battery is that if the electrolyte uses an organic electrolyte commonly used for lithium-ion batteries, the battery capacity will be significantly reduced with the charge-discharge cycle. The sulfur-lithium intermediate compound produced during the charge-discharge reaction of the battery dissolves in the electrolyte and reacts on the negative electrode side, resulting in a large reduction in the amount of sulfur used for charge and discharge.
Change the electrolyte or carbon material
One of the countermeasures considered in this regard is the use of a solid electrolyte that is more stable than liquid to prevent the elution of sulfur. Northeastern University’s R&D team is developing complex hydrides that can be used in such solid electrolytes.
The R&D team focused on complexing hydrides because of its higher stability when used in batteries. Uchihara said, "Before the sulfides and oxides were widely studied as solid electrolytes. Although there are very high ionic conductivity types that can be used in batteries, there are not many types of stability required for battery operation."
The complex hydride means that the metal cation M (Li+, Na+, Mg2+, etc.) and the complex anion (M'H)n [(BH4)-, (NH2)-, (AlH4)-, (AlH6)3-, etc. ] Composition of M(M'H)n substances. It is also not easily thermally decomposed at a high temperature of 150°C. Light elements can be used as the constituent elements, and a precise electrolyte can be produced only by uniaxial pressing at room temperature. However, the ionic conductivity is low and the operating temperature is high.
For example, the current electrolyte ion conductivity is 10-2 S/cm or more (room temperature). Lithium borohydride (LiBH4), which is one of complex hydrides, has an ionic conductivity of 2×10-3S/cm or more at a temperature of 390K (about 120°C) and about 10-7S/cm at room temperature (Figure 1). ,2). The R&D group increased the ionic conductivity at room temperature to about 10-5 to 10-4 S/cm by replacing part of the BH4 ion [(BH4)-] with iodide. However, Yu said, "To achieve the same level of energy density and output density as current lithium-ion batteries, it needs to be increased to about 10-3 S/cm." In addition to LiBH4, this research team is also exploring other various complex hydrides. Li2B12H12 (about 10-4 S/cm at 60°C) and compounds such as LiNH2 and LiBH4 are also candidates.
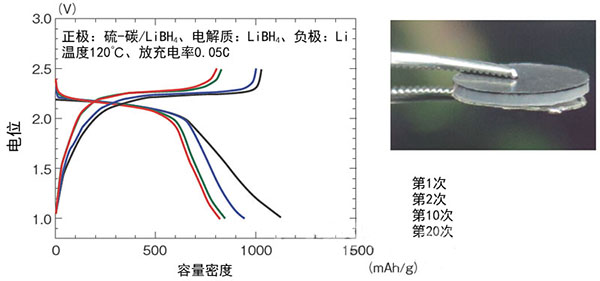
Figure 1: Performance evaluation of a prototype solid all-solid lithium-sulfur battery
Professor of Tohoku University was developed by R&D team of Fushio et al. The cathode uses sulfur and the capacity density is as high as 800 mAh/g (20th time).
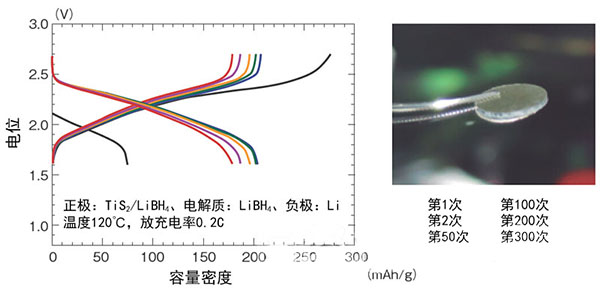
Figure 2: Performance evaluation of a trial-made bulk solid-alloy TiS2/Li battery
Professor of Tohoku University was developed by R&D team of Fushio et al. The positive electrode adopts TiS2, and it can charge and discharge 300 times or more repeatedly with 0.2C.
In fact, the R&D team developed a basic technology for lithium-ion batteries that can be used in high-temperature engine compartments in cooperation with Hitachi. This technology is used in lithium-ion batteries and maintains a theoretical capacity of 90% at a high temperature of 150°C (Fig. 3).
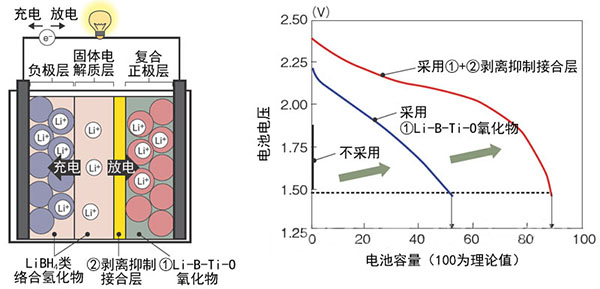
Figure 3: Basic Technology for Implementing High-Temperature Lithium-Ion Batteries
Professor Tohoku University's R&D team is jointly developed with Hitachi. Left is the battery structure. Right is the relationship between battery voltage and battery capacity. Using the new technology (1+2) ensures that the theoretical capacity is 90% of the battery capacity.
The important point is that by sandwiching the mixture layer of the complex hydride LiNH2 and LiBH4 between the positive electrode layer and the solid electrolyte layer, the peeling phenomenon accompanying the volume change at the time of charging and discharging between them is prevented. In addition, Li-BTi-O (lithium-boron-titanium-oxygen)-based oxides are used as a binder for a three-way active material used as a positive electrode material, and a decomposition reaction of the positive electrode material in contact with LiBH4 is prevented.
In addition, to prevent the sulfur of LIS cells from leaching out, the research and development team led by Shi Chuan Zheng Division and Associate Professor Yaji Yamashi of the Department of Chemical and Life Sciences at Kansai University developed a method to change the carbon material that adsorbs sulfur using positive electrodes instead of electrolytes. Yamagata stated that "if a carbon material with a pore diameter of 1 nm or less is used, sulfur in the pores does not easily come out," thereby preventing sulfur from decreasing with the charge-discharge cycle.
In addition to this carbon material, the research and development team also increased the output power of LIS batteries by using sodium alginate as a sulfur cathode binder. Use of sodium alginate - magnesium alginate as a binder. The sulfur-activated carbon composite active material and the conductive auxiliary acetylene black were solidified together with magnesium alginate to prepare a positive electrode material. Lithium metal as the negative electrode material and an ionic liquid using an electrolyte (only low-melting-point salt composed of cation and anion plasmas) was used as the prototype LIS cell. High-capacity of about 900 mAh/g was maintained after 15 cycles of 0.5 C charge and discharge. The use of other ionic liquids as the electrolyte, as well as the ability to maintain a high capacity of approximately 900 mAh/g after 70 cycles of charge and discharge, and as a type of battery operation at 2.0C. (Reporter: Tomihiro Miooka)
The Magnifying Makeup Mirror is a specialized mirror designed for applying makeup with precision. It typically features a magnification factor of up to 10 times the normal size, allowing for close-up views of small areas such as the eyes or lips. Some models may also include adjustable lighting to provide optimal illumination.
Magnifying Makeup Mirror, Bath Makeup Mirror, Bathroom Magnifying Mirror
Jiangmen Yunspire Sanitary Ware Hardware Industry Co., Ltd. , https://www.yunspirefaucet.com